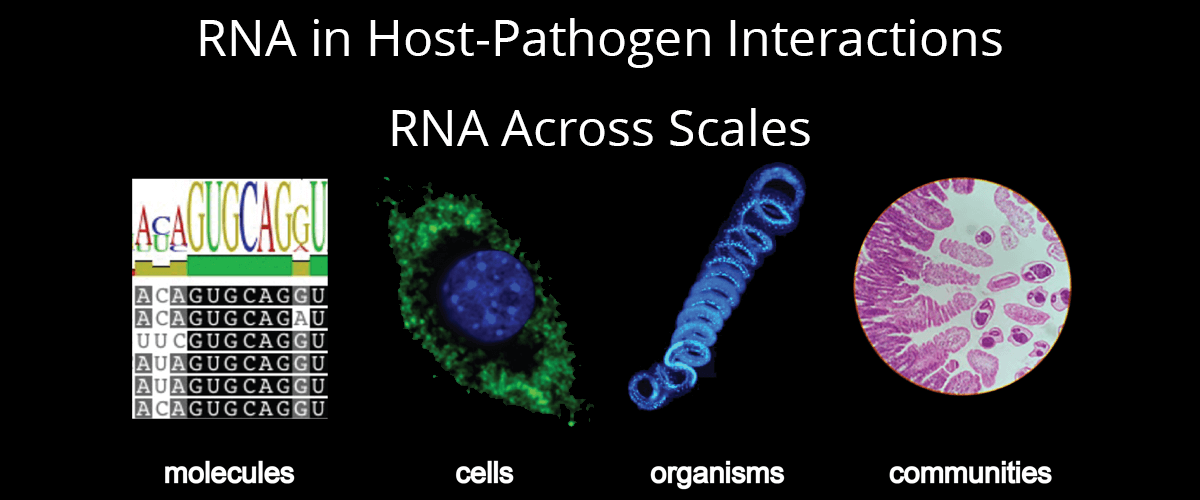
Host-pathogen interactions form the basis for numerous therapeutic, vaccine and diagnostic strategies in infectious disease. Development of these strategies requires an in-depth understanding of the molecules used by host and pathogen to control infection and/or evade the counter-response. Research in this area has historically concentrated on proteins, lipids, or small molecules. Research in our group focuses on the role of another type of molecule, ribonucleic acid (RNA), in host-pathogen interactions.
Small pieces of RNA control the quantity of individual proteins expressed at a given time in a cell. During infection these pieces of RNA can therefore determine how well an organism can mount an immune response to a pathogen. Our research focuses on the functions of these small RNAs in the response to viruses and other pathogens. We examine how viruses interact with small RNAs and we are testing how modulating the levels of specific small RNAs can alter the ability of viruses to grow and spread.
We also study how small RNAs can move from one cell to another – for example from a virus-infected cell to a neighbour cell or from an extracellular parasite into a host cell. We are currently working to understand these natural processes of RNA secretion and uptake, in particular during gastrointestinal nematode infections. Because pathogen-derived small RNAs can be detected in human body fluids, our research also examines the diagnostic utility of extracellular RNAs in infectious diseases (see Collaborations).
RNA Communication
Small RNAs have yielded many new insights into host-pathogen interactions, and the pathogen models have also helped us understand new fundamental aspects of RNA biology. In particular, our lab is investigating a new field in RNA biology—RNA communication. We think of this as the ability of RNA to transmit information between different genomes, cells, organisms and species.
The primary mechanism of gene regulation that we study is RNA interference (RNAi), where two different RNAs interact within an RNA-induced silencing complex (RISC) to mediate epigenetic, transcriptional or post-transcriptional gene regulation. In some organisms, including nematodes, the gene regulation mediated by RISCs is transmitted between generations (trans-generational epigenetic inheritance). In multi-organism systems (such as the gut) components of RISCs can also be transmitted between cells, organisms or species as a form of communication. We use host-pathogen systems to characterise these “trans-genome” RNA interactions, in order to understand the function and mechanism of RNA communication in living systems.
Viral-Host Interactions
Different viruses have evolved to produce short or long RNAs that enter host RISCs and impact gene expression. As intracellular obligate parasites, viruses provide unique models to understand how foreign RNAs can enter host RNAi pathways, how the RNAi pathways are regulated and how RNAi signals are transmitted from one cell to another. We are currently studying the viral-host RNA interactions that enable signalling from infected to uninfected cells in herpesvirus and respiratory virus infection.
Cross-Species RNA Interference
The mammalian gut is a complex ecosystem of different organisms that communicate to share resources, coordinate digestion and maintain homeostasis. In many animals, parasitic nematodes are part of this ecosystem, where they promote tolerance and modulate the local environment to favour their survival. We have found that gastrointestinal nematodes release both RNA and protein components of RISCs (Argonaute proteins) to modulate host cells and the gut environment. We want to understand how RNA transmission from a nematode to a mammal works and what role RNA communication plays in gut homeostasis, infection and inflammation.
Cell-to-Cell Communication
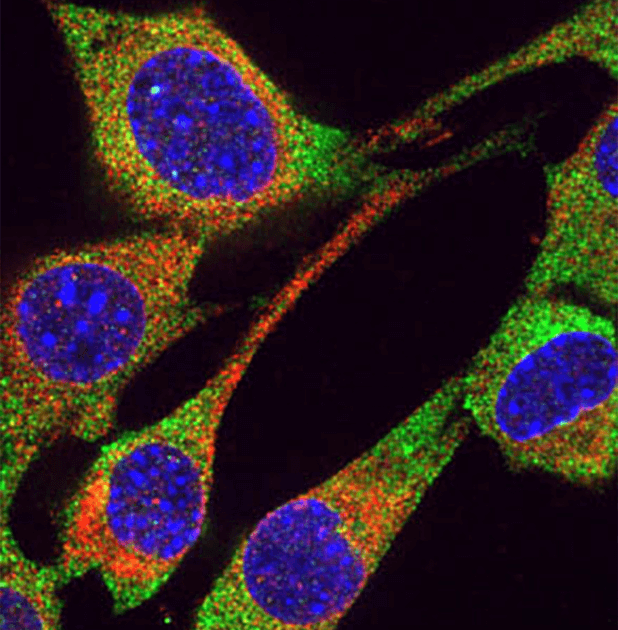
We want to understand the properties of RNA that is naturally transmitted between mammalian cells as a communication mechanism. We are using biochemical and genetic strategies to understand how RISC components (both RNAs and proteins) can be transmitted from one cell to another (inside or outside of extracellular vesicles) in mammals. Ultimately, we want to understand the evolution of RNA-based communication, its function inside organisms, as well as its role in enabling complex multi-organism communities.